The Patient Safety Group (PSG) of the Royal College of Surgeons of Edinburgh (RCSEd) are delighted to lend our enthusiastic support to the sixth World Patient Safety Day (WPSD). This event, established by the World Health Organisation (WHO) in 2019, takes place on 17 September every year. It helps to raise global awareness amongst all stakeholders about key Patient Safety issues and foster collaboration between patients, health care workers, health care leaders and policy makers to improve patient safety. Each year a new theme is selected to highlight a priority patient safety area for action.
The theme set by the WHO for this year’s WPSD is “Improving diagnosis for patient safety”, recognising the vital importance of correct and timely diagnosis in ensuring patient safety and improving health outcomes.
Introduction
Diagnostic error has been defined as the failure to (a) establish an accurate and timely explanation of a patient’s health problem(s) or (b) communicate that explanation to the patient (1). Failure in the diagnostic process, leads to diagnoses that are delayed, incorrect, or even missed altogether.
Diagnostic error is one of the most important safety problems in health care today, and inflicts the most harm, dwarfing all other causes of harm from medical errors combined. It is estimated that one in three patients experience a diagnostic error at some point in their treatment (2). Surgical malpractice claims data from Canada estimate that 16% are related to errors in diagnosis (3).
Safe, accurate, efficient and timely diagnosis is the foundation on which all future decisions about treatment and prognosis are made. It is crucial to all phases of surgical care and there is a significant impact if errors are made. If an error is made at this initial stage, then the ramifications extend through the entire patient journey. It is well recognised that diagnostic errors – not surgical mistakes – account for the most severe patient harm. 54% of surgical patients who experience a diagnostic error go on to suffer at least moderate harm, with one in seven dying as a result (3).
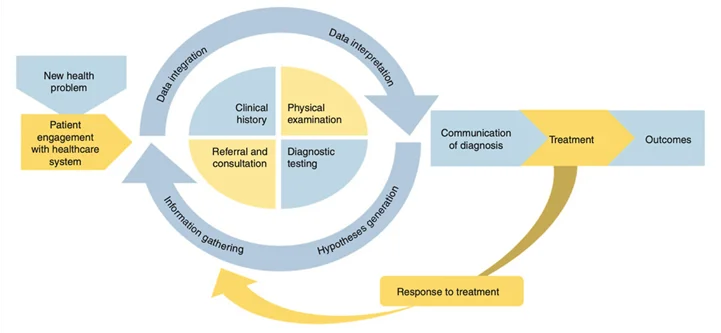
The Surgical Diagnostic Pathway
Singh has described the five interactive process dimensions of diagnosis (4). These include:
- Patient-clinician encounter (history, physical examination, ordering of tests, referrals based on assessment)
- Performance and interpretation of diagnostic tests
- Follow up and tracking of diagnostic information over time
- Subspecialty and referral-specific factors
- Patient-related factors
Availability of clinical data that provide a longitudinal picture across care settings is essential to understanding a patient’s diagnostic journey.
Challenges to Safe Surgical Diagnosis
The surgical diagnostic process is highly complex, involving uncertainty and susceptibility to errors. It is multifaceted, unfolds over time and often across many episodes of care with different members of the healthcare team in the context of a highly complex healthcare system. Multiple handovers in care are often required. It continues to evolve while transitions in care occur, as new information is added, test results return, and different providers join the team, bringing their own perspectives and diagnostic skills.
Accuracy is often poorly defined, with no widely accepted standards for how long a diagnosis should take for most conditions, particularly in the face of unusual presentations and/or rare conditions. It can therefore be difficult to even determine what is accurate and timely and if an error definitely occurred. Patient presentations often evolve over time, and sometimes the best approach is to defer diagnosis to a later date when more information is available or if symptoms persist or evolve.
Furthermore, many symptoms have no underlying diagnosis despite best practice. Diagnostic pathways are highly variable and cannot easily be standardised across different diseases and settings.
Harms from diagnostic errors often do not happen in real time so clinicians are not always aware. Even when these errors are uncovered, analysis and learning are limited, resulting in inadequate performance feedback to individual clinicians.
Diagnostic errors are hard to measure; they are complex, multifactorial events that can happen during any encounter with the health system. There is a lack of robust research in the area, with no reliable valid guidelines as to what correct diagnosis looks like, no standardised tools by which diagnostic safety can be measured and reported, and no clear mechanism of feeding back diagnostic errors to the healthcare team (5).
Causes of Surgical Diagnostic Error
Diagnostic errors are shaped by complex dynamics involving patient, clinician, team and system-related factors.
The diagnostic process is not limited to the patient-provider interaction, and errors may result from the complex interplay of parameters related to patients (e.g. health literacy, presenting symptoms, complexity, co-morbidity and behaviours), provider-care teams (e.g. the cognitive load on providers, information gathering and synthesis), and systems (e.g. health information technology, workflow design, crowding, time pressure, shift work and interruptions) (6).
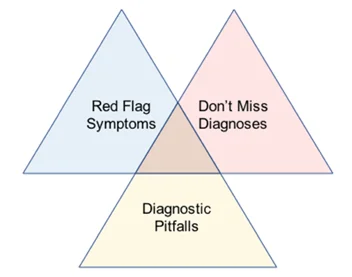
Olson proposes a tripartite diagnosis framework. Diagnostic safety depends on not missing worst case scenario diagnoses and identifying important red flags, clues that often signify serious pathology. Being alert to common diagnostic pitfalls is also key (diagnostic mimics, atypical presentations, false positive/negative investigation results) so that situation awareness can be improved, and these can be avoided. System factors affect ability to address these three issues (7).
The likelihood of diagnostic error varies between conditions and specialties. For example, colorectal and other cancers are often missed, whereas conditions based on visual clues and pattern recognition, e.g. dermatologic or radiologic conditions, are much less effected by error.
Diagnostic errors happen across all clinical care settings and with all diseases. However, most serious misdiagnosis-related harms are concentrated in three major disease categories - vascular events, infections, and cancers - which account for 75% of serious harms, and just fifteen diseases account for half of serious misdiagnosis-related harms (8).
There is an increased risk for diagnostic errors in vulnerable patient groups due to implicit bias based on race, gender, or mental health problems (9).
Malpractice claims data reveal that diagnostic error occurs in most surgical specialties with general surgery (39%), gynaecology (18%), orthopaedic surgery (12%), urology (10%), and plastic surgery (7%) contributing most cases. Surgical diagnostic errors occur most frequently in the inpatient setting (61%), occurring about half as often in the outpatient setting (31%) (3)
Surgical care often requires the unique ability to make rapid, split-second decisions to save a patient’s life, limb, or vital organs. Although this skill set is essential for surgeons, this type of decision-making is most vulnerable to faulty heuristics and cognitive errors (10).
More than 80% of factors contributing to diagnostic errors in surgical patients are attributed to clinicians, with clinical decision-making (e.g. deficient assessment, failure to perform a necessary test or intervention, misinterpretation of a test, failure to refer) being the primary contributing factor. Inadequate evaluation of a presenting condition or comorbidity, failure to follow-up on a complication and loss of situational awareness (e.g. inadequate monitoring or follow-up, insufficient knowledge or skill, failure to review medical record, premature discharge from hospital) are additional clinician contributing factors (3). Surgeons may have limited experience with uncommon diseases and not recognise important patterns.
50% of factors contributing to surgical diagnostic errors are attributable to health care team factors, the most common of which is communication breakdown with the patient (e.g. inadequate communication while obtaining informed consent, inadequate communication at discharge, inadequate disclosure of error) or between clinicians (e.g. inadequate handover of care), and issues related to documentation (e.g. inadequate detail in documentation of care provided).
12% of factors contributing to diagnostic errors in surgery have been attributed to the broader health care system. Resource issues (e.g. malfunctioning equipment, insufficient or unavailable resources, waiting times) and procedural issues (e.g. inadequate administrative procedure, test result mix-up) have been identified as the commonest contributing factors (3).
Pre-operative Phase
33% of diagnostic errors in surgery occur in the pre-operative phase. Common diagnostic pitfalls in this phase include inaccurate and delayed review of pathology results, failure to confirm a diagnosis prior to proceeding with emergency surgery, failure to diagnose serious conditions in a timely manner resulting in treatment delays.
Cancer (mostly frequently lung and connective tissue primary cancer) is the most common diagnosis involving errors in the pre- operative phase. Delayed diagnosis of cancer can be a major cause of harm. The situation is challenging as there are few robust guidelines on making a timely diagnosis; many cancers often present with non-specific features early in their course and aren’t suspected until symptoms persist or worsen (3).
Intra-operative Phase
31% of diagnostic errors in surgery occur in the intra operative phase, with limitations being encountered in the identification of the relevant surgical pathology, unconventional anatomy, intraoperative injury or retained foreign bodies (3).
Post-operative Phase
The majority of diagnostic errors in surgery (44%) occur in the post operative phase of care. These include timely recognition of clinical deterioration, accurate identification of postoperative sepsis, together with other significant complications such as anastomotic leak and cancer progression, and failure to arrange appropriate follow up (3).
Surgical Outpatient Clinic
The fragmented environment of outpatients when a patient’s diagnostic process takes place over time and multiple visits is at particularly high risk of diagnostic error.
Diagnostic process breakdowns occur frequently in this setting and include failure to obtain an adequate family history in 38% of cases, perform an appropriate physical exam in 23%, and order appropriate laboratory tests in 16% (11). Key pieces of information (such as history or test results) documented in the medical record may not be followed up (12), red flags (such as new anaemia or rectal bleeding) may not be acted upon (13).
These challenges are now potentially compounded by virtual consultations which are challenged by the lack of a face-to-face encounter and physical examination (14).
Emergency Surgical Service
Diagnostic safety in the emergency surgical admission setting is also particularly challenging and the risk of erroneous decision making is high (15). Various stakeholders make crucial medical decisions in a time-pressured often chaotic, resource constrained environment often with incomplete information. The situation is further compounded by frequent interruptions, care handoffs, overcrowding, and boarding. Emergency patients are often in extreme pain or distress, impacting on their ability to pass on key information about their medical history.
Diagnosing Frailty in Surgical Patients
The elderly population is rapidly growing and represents an ever-increasing proportion of our surgical patients. Frailty has repeatedly been shown to be a powerful predictor of poor surgical outcome, being associated with higher mortality, increased rates of postoperative complications, longer hospital stays, reduced independence and higher rates of discharge to assisted living facilities (16). Furthermore, optimising a frail patient’s functional status pre-operatively translates into increased intra-operative and post-operative robustness.
It is vital to accurately diagnose frailty in the pre-operative period so that measures can be put in place to optimise both pre-operative preparation and the support available following surgery. Frailty diagnosis allows for targeted interventions aimed at improving post-operative outcomes (e.g. medication reconciliation, nutrition counselling and exercise programs). It also facilitates more meaningful multidisciplinary discussion of goals of care with patient and their families, considering how a patient’s frailty status will affect their outcomes. Furthermore, pts can be enrolled in frailty pathways, facilitating a multidisciplinary approach to peri-operative care. Rehabilitation and discharge planning are streamlined (17).
Risk Stratification
Correct diagnosis of co-morbidity in the pre-operative period allows for more informed discussion of risks, better risk stratification and identification of those for whom surgical procedures will not be beneficial in terms of survival or improved quality of life. It also allows for better optimization of co-existing diseases and use of pre-operative interventions such as medication optimization, psychological support, physical exercise program, nutritional interventions, advice on stopping smoking, advice on losing weight (18).
How to Improve Surgical Diagnostic Safety
The diagnostic process is embedded in the work system, so getting it right involves tackling a wide range of interrelated issues (19). We need to ensure surgical diagnosticians are equipped with the necessary factors - time, tools, teams, training and technologies - to make high-quality, safe diagnostic decisions. Systems need to be in place to allow clinicians to get the diagnosis right even when time is short (20).
At an individual clinician level, general strategies to improve diagnostic performance include seeking feedback on diagnostic decisions, integrating short diagnostic challenge rehearsals into daily routine, considering cognitive biases, fostering critical thinking skills. Tunnel vision and confirmation bias must be avoided and clinicians must be prepared to consider alternative diagnoses. Diagnoses should be considered working hypotheses and not set in stone (21). Situational awareness of local, disease-specific, literature-reported vulnerabilities and pitfalls is also important.
At a systems level, diagnostic errors can be reduced by improving diagnostic decision support systems, improving the design and functionality of electronic health records.
Proactive, reliable follow-up safety nets and feedback systems to detect and protect should be implemented. Test results must be read and acted upon in timely manner. Closed loop systems should be in place to ensure that every test result is returned, acknowledged, and acted on as well as communicated to the patient by the ordering clinician (22).
Teamwork is crucial if correct diagnoses are to be made. Input is required from a wide multidisciplinary team involving frontline clinicians, nurses as well as laboratory, pathology and radiology staff (23). There is a need to improve integration, coordination, and communication between clinical teams and patients/caregivers across the diagnostic continuum. Particular focus is required on transitions of care and improved more structured handover protocols are vital. Interventions intended to improve teamwork or standardise communication using checklists, proformas, and information technology have shown potential to improve surgical communication (24).
Furthermore, it is absolutely crucial to harness the expertise of patients and their families, listening actively to their concerns and communicating effectively. Patients hold critical knowledge about their health that informs the diagnostic process and bear the ultimate risk of harm from diagnostic error (25). They have alternative perspectives on health care delivery when receiving care and observing clinical staff. They are, at times, the only connecting thread between various encounters with different providers or health care systems. With their unique perspective, they are also in a perfect position to more easily identify errors in their records (26).
By accessing their own health information, patients can see the clinician’s perspective, identify and correct missing or inaccurate information or misunderstandings before they can cause harm, and strengthen shared mental models with clinicians.
Sharing visit notes also helps engage patients/ families as diagnostic partners, improve their retention of information, improve attendance at diagnostic tests and onward referrals, and improve relationships with their providers (27). Access to notes also enables patients/ families to learn more about their health conditions, formulate questions without the time pressure or stressors of the clinical environment, and participate as informed members of the health care team (28). Furthermore, patients who experience diagnostic error can provide a unique perspective on what is needed for diagnostic error reduction and can help inform priorities for the research agenda.
There has been increasing recognition over recent years of the importance of listening to the patient and taking on board family concerns. Martha’s rule has been implemented in the NHS in England to facilitate access to a second opinion should relatives be concerned about clinical deterioration (29). In the US patients now have federally mandated access to electronic health information via the 2021 Cures Act Final Rule.
It is important to balance timely and accurate diagnosis with overuse of tests, over diagnosis, over treatment, over utilisation of scarce resources and increased patient exposure to risk from invasive tests. For example, if CTPA was ordered for every patient with suspect pulmonary embolus the missed diagnosis rates would reduce but there would be increased risk of radiation exposure, false positives, incidental findings and high cost. It is also important to avoid over diagnosis, detection of a disease that would have never caused harm in a patient’s lifetime (e.g. indolent cancers detected only through screening tests and over detection of irrelevant findings, such as thyroid nodules found on CT scans ordered for other indications (30). Risk prediction calculators can be useful in these settings to help find a balance between over and under diagnosis (18).
Qualitatively understanding the plethora of diagnostic errors locally and across institutions can help us build the situational awareness and safety nets we need for better diagnostic conduct. Frontline clinicians and patients offer unique perspectives in identifying pitfalls in the diagnostic process as they can share personal insights and their lived experiences with making and receiving diagnoses in this setting (31).
It is important to foster a culture of diagnostic safety and improvement, replacing blame and fear with learning and improvement. The hierarchy should be levelled, and all staff should be empowered to identify and report diagnostic errors and improvement opportunities. There should be an organisational commitment to improving diagnostic safety, celebrating and disseminating diagnostic safety success, and championing diagnostic safety in policies and practices. Standardised and reliable methods of identification, measurement and reporting of diagnostic safety should be developed and implemented. Routine structured evaluation and feedback on diagnostic safety should be regularly provided to teams. Training should be provided to all staff on principles of diagnostic safety (32).
Tools
Over recent years several tools have been developed to help generate learning and improvement opportunities in diagnosis, for use by front-line clinical teams, patients and health care organisations. Examples of these include:
Clinicians and Teams
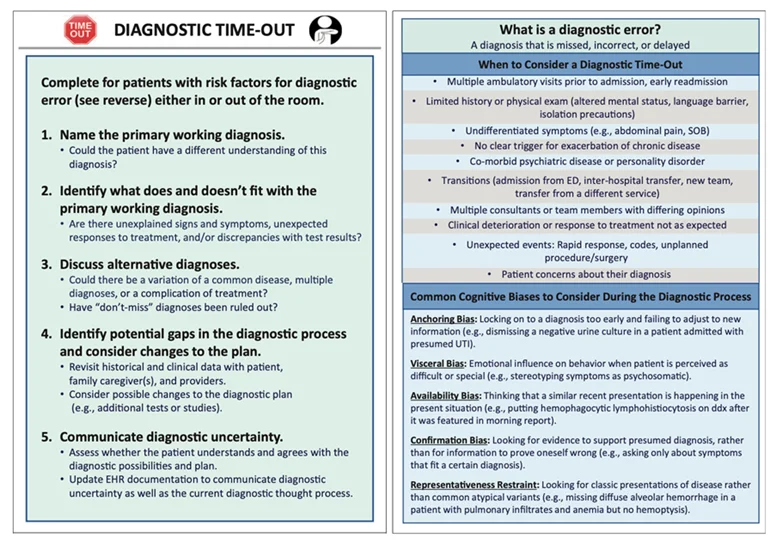
Diagnostic Time Out: A resource for individual clinicians to reassess the working diagnosis. It prompts the clinical team to pause and re-evaluate the working diagnosis and is akin to the Surgical Safety Checklist now in widespread use to improve operating room safety (33).
alibrate Dx: a resource for individual clinicians to self-assess their diagnostic performance by reviewing samples of their recent clinical practice (34).
TeamSTEPPS for Diagnosis Improvement: a course for individuals and teams that applies evidence-based communication and teamwork skills to improving diagnosis (34).
Patients
Patient Diagnosis Questionnaire: a web-based survey which allows patients to report their understanding of the diagnosis and satisfaction with communication from their clinical team (33).

The Toolkit for Engaging Patients to Improve Diagnostic Safety: provides strategies to improve communication within the patient- physician encounter (34).
OurDx: an online tool to better engage patients and families in the ambulatory diagnostic process, shown to be particularly beneficial in reducing diagnostic error in patients from racial minorities (9).
Systems
Electronic Health Record (EHR):
Recognition of key red flags in EHRs can be improved by capturing essential data (e.g. family history of cancer, abnormal test results) in a more structured way so the data can be used for improving care. Similar technology can be used to build back-up systems to catch abnormalities that might have otherwise been missed (33).
Trigger tools:
Electronic trigger (e-trigger) tools, which mine vast amounts of patient data to identify signals indicative of a likely error or adverse event, offer a promising method to efficiently identify errors. Such tools allow identification of patients at high risk for diagnostic error so that their medical records can be selectively reviewed (35).
Safer Diagnosis e-trigger tools detect potential diagnostic events, allowing health systems to monitor event rates, study contributory factors and identify targets for improving diagnostic safety. In addition to promoting organisational learning, some e-triggers can monitor data prospectively and help identify patients at high risk for a future diagnostic error so that their medical records can be reviewed enabling clinicians, patients or safety personnel to take proactive preventive action.
Triggers that have been utilised include patient deterioration, escalation of care, unexpected time course of illness, change in management plan and diagnostic uncertainty. These tools have helped to identify patients with abnormal CT scans, prostate-specific antigen values, rectal bleeding, and positive faecal occult blood tests who had not been followed up on a timely basis (36). They have also been found to be effective in reducing time to diagnostic evaluation of colorectal and prostate cancer and improving the proportion of patients who receive follow-up as compared to usual care (37).
Early Warning Scoring (EWS) systems to help diagnose deterioration:
EWS are simple track and trigger tools to identify hospital patients at risk of deterioration prompting a warning so that care can be escalated appropriately, reducing unnecessary harm (38). One commonly used standardised example is the National Early Warning Score (NEWS) developed by the Royal College of Physicians in 2012 (39) and modified in 2017 (NEWS2) (40).
Artificial Intelligence (AI):
In recent years, AI has shown promise in automating steps in the diagnostic process. These advances help prioritise cases requiring expert clinician review, thereby revolutionising how we arrive at diagnoses. Examples include using machine learning algorithms to flag potential cases of intracranial haemorrhage on CT (41), and AI-assisted autocomplete systems to increase the quality and efficiency of documentation and data entry (42).
Organisation-level
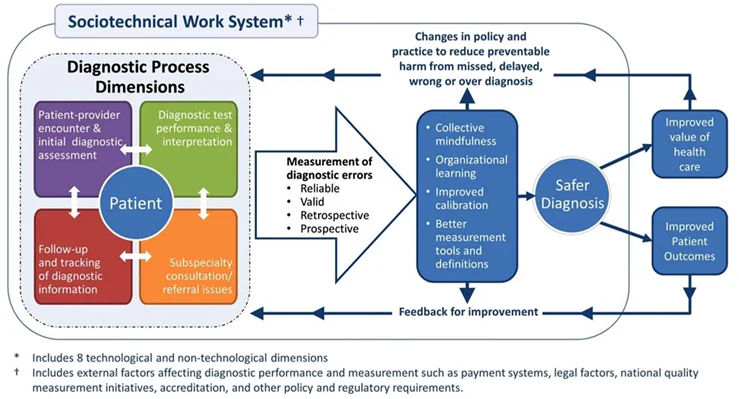
Safer Dx Framework:
A self-assessment tool outlining ten recommended practices that can be used to understand current organisational diagnostic safety procedures and identify opportunities for improvement (43). It provides a conceptual foundation for system-wide safety measurement, monitoring and improvement.
This continuous feedback loop includes the complex adaptive socio-technical work system in which diagnosis takes place (structure), the process dimensions in which diagnoses evolve beyond the clinician’s visit (process), and resulting outcomes of “safe diagnosis,” (i.e. correct and timely), as well as patient and healthcare outcomes (outcomes). The Safer Dx framework underscores that diagnostic errors can emerge across multiple episodes of care and that corresponding clinical data across the care continuum are essential to inform measurement.
Priorities include targeting leadership (a board-to-bedside accountability framework) and learning health systems (deliberate feedback loops on diagnostic out- comes; dedicated multidisciplinary learning teams), a focus on diagnostic equity, transitions of care, and the critical role of patients and families on the diagnostic team and in the diagnostic learning system.
Undesirable Diagnostic Events (UDEs) Measurement
Olson et al have proposed a framework, based on principles like those of trigger tools, for identifying UDEs. Conditions prone to diagnostic error and the contexts of care in which these errors are likely to occur are identified. Refinement and adoption of this framework across health systems can improve standardised measurement and reporting of diagnostic safety (44).
Measure Dx: a resource for clinicians and organisations to detect, analyse, and learn from diagnostic safety events (34).
Written by Anna Paisley, Consultant General Surgeon, RCSEd Council Member and Patient Safety Group Chair
- The National Academies of Sciences, Engineering and Medicine (NASEM) report Improving Diagnosis in Healthcare (National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://doi.org/10.17226/21794.
- Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf 2013 Oct;22 Suppl 2:21-27.
- Kwan JL, Calder LA, Bowman CL et al. Characteristics and contributing factors of diagnostic error in surgery: analysis of closed medico-legal cases and complaints in Canada. Can J Surg 2024; 67(1):E58-65.
- Singh H, Bradford A, Goeschel C. Operational measurement of diagnostic safety: state of the science. Diagnosis 2021; 8(1): 51–65.
- Newman-Toker DE, Austin JM, Derk J et al. Are health care provider organizations ready to tackle diagnostic error? A survey of Leapfrog-participating hospitals. Diagnosis 2017;4:73-78.
- Bergl PA, Wijesekera TP, Nassery N et al. Controversies in diagnosis: Contemporary debates in the diagnostic safety literature. Diagnosis 2020; 7(1): 3-9.
- Olson APJ, Linzer M, Schiff GD. Measuring and improving diagnostic safety in primary care: addressing the “twin” pandemics of diagnostic error and clinician burnout. J Gen Intern Med 2021; 36(5):1404–6.
- Newman-Toker DE, Wang Z, Zhu Y et al. Rate of diagnostic errors and serious misdiagnosis-related harms for major vascular events, infections, and cancers: toward a national incidence estimate using the “Big Three”. Diagnosis 2019; 6(3): 227–240.
- Bourgeois FC, Hart NJ, Dong Z et al. Partnering with patients and families to improve diagnostic safety through the OurDX Tool: effects of race, ethnicity, and language preference. Appl Clin Inform 2023;14:903–912.
- Hughes TM, Dossett LA, Hawley ST, et al. Recognizing heuristics and bias in clinical decision-making. Ann Surg 2020;271:813-4.
- Weingart SN, Saadeh MG, Simchowitz B et al. Process of care failures in breast cancer diagnosis. J Gen Intern Med. 2009;24(6):702–709.
- Singh H, Meyer AND, Thomas EJ. The frequency of diagnostic errors in outpatient care: estimations from three large observational studies involving US adult populations. BMJ Qual Saf. 2014;23(9):727-731.
- Singh H, Daci K, Petersen L et al. Missed opportunities to initiate endoscopic evaluation for colorectal cancer diagnosis. Am J Gastroenterol 2009;104(10):2543–2554.
- Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc 2020;27(6):957-962.
- Enayati M, Sir M, Zhang X et al. Monitoring diagnostic safety risks in emergency departments: protocol for a machine learning study. JMIR Res Protoc 2021; 10(6):e24642.
- Li JL, Henderson MA, Revenig LM et al. Frailty and one-year mortality in major intra-abdominal operations. J Surg Res. 2016;203:507.
- Acosta A, Garzon MP, Urman RD et al. Screening and diagnosing frailty in the cardiac and non cardiac surgical patient to improve safety and outcomes. Int Anaes Clinics 2019;57(3):111-122.
- Mansmann U, Rieger A, Strahwald B et al. Risk calculators-methods, development, implementation, and validation. Int J Colorectal Dis 2016; 31:1111–6.
- Marsh KM, Turrentine FE, Knight K et al. Defining and studying errors in surgical care. A systematic review. Ann Surg 2022;275:1067–1073.
- Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. B MJ 2022;376:e068044 http://dx.doi.org/10.1136/bmj-2021-068044
- Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf 2013;22:ii65-72.
- Wright B, Lennox A, Graber ML, et al. Closing the loop on test results to reduce communication failures: a rapid review of evidence, practice and patient perspectives. BMC Health Serv Res 2020; 20(1):897.
- Greenberg CC, Regenbogen SE, Studdert DM, et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg 2007;204:533-40.
- Nagpal K, Vats A, Lamb B, et al. Information transfer and communication in surgery: a systematic review. Ann Surg 2010; 252:225-39.
- Wyner D, Wyner F, Brumbaugh D, et al. A family and hospital’s journey and commitment to improving diagnostic safety. Pediatrics 2021;148(6):e2021053091
- Bell SK, Delbanco T, Elmore JG, et al. Frequency and types of patient-reported errors in electronic health record ambulatory care notes. JAMA Netw open. 2020;3(6):e205867-e205867.
- Gandhi TK, Kaplan GS, Leape L, et al. Transforming concepts in patient safety: A progress report. BMJ Qual Saf. 2018;27(12):1019-1026.
- Fossa AJ, Bell SK, DesRoches C. OpenNotes and shared decision making: a growing practice in clinical transparency and how it can support patient-centered care. J Am Med Inform Assoc 2018; 25(09):1153–1159.
- Cooksley, T, Astbury, S, Holland, M. Martha’s rule and patient rights to a second opinion. BMJ 2023;383:2221-2221.
- Carter SM, Rogers W, Heath I et al. The challenge of overdiagnosis begins with its definition. BMJ 2015;350:h869.
- Courtney WM, James TG, Parker SJ et al. Frontline providers’ and patients’ perspectives on improving diagnostic safety in the emergency department: a qualitative study. Jt Comm J Qual Pat Safety 2024;50:480-91.
- Schiff GD, Ruan EL. The elusive and illusive quest for diagnostic safety metrics. J Gen Intern Med 2018;33(7):983–5.
- Garber A, Garabedian P, Wu L et al. JAMIA Open; 2023; 6(2), ooad031 https://doi.org/10.1093/jamiaopen/ooad031
- Bradford A, Goeschel C, Shofer M et al. Five New ways to advance diagnostic safety in your clinical practice. American family Physician 2023;108(1):14-6.
- Murphy DR, Meyer AN, Sittig DF, et al. Application of electronic trigger tools to identify targets for improving diagnostic safety. BMJ Qual Saf 2019;28:151-9.
- Shenvi EC, El-Kareh R. Clinical criteria to screen for inpatient diagnostic errors: a scoping review. Diagnosis 2015;2:3–19.
- Murphy DR, Wu L, Thomas EJ et al. e trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol 2015;1(3);3560-67.
- Patel R, Nugawela MD, Edwards HB et al. Can early warning scores identify deteriorating patients in pre-hospital settings? A systematic review. Resuscitation 2018:132;101–111.
- Royal College of Physicians. National Early Warning Score (NEWS): standardising the assessment of acute-illness severity in the NHS. Report of a working party. London: RCP, 2012.
- Royal College of Physicians. National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Updated report of a working party. London: RCP, 2017.
- Titano JJ, Badgeley M, Schefflein J et al. Automated deep-neural-network surveillance of cranial images for acute neurologic events. Nat Med 2018;24:1337–41.
- Greenbaum NR, Jernite Y, Halpern Y et al. Improving documentation of presenting problems in the emergency department using a domain-specific ontology and machine learning-driven user interfaces. Int J Med Inform. 2019;132:103981.
- Singh H, Mushtaq U, Marinez A, et al. Developing the Safer Dx Checklist of ten safety recommendations for health care organizations to address diagnostic errors. Jt Comm J Qual Patient Saf. 2022;48(11):581-590.
- Olson APJ, Graber ML, Singh M. 2018. Tracking progress in improving diagnosis: a framework for defining Undesirable Diagnostic Events. J Gen Intern Med 2018;33(7):1187–91.

